Review of Headã¢â‚¬âtoã¢â‚¬âhead Comparisons of Glucagonã¢â‚¬âlike Peptideã¢â‚¬â1 Receptor Agonists
- Original investigation
- Open Access
- Published:
Indirect comparison of glucagon like peptide-1 receptor agonists regarding cardiovascular safety and bloodshed in patients with type two diabetes mellitus: network meta-analysis
Cardiovascular Diabetology volume 19, Article number:96 (2020) Cite this commodity
Abstract
Background
The cardiovascular upshot trials (CVOTs) take shown that glucagon like peptide-i receptor agonists (GLP1RAs) have varying degrees of cardiovascular (CV) safety in patients with type two diabetes mellitus (T2DM.) The lack of any head-to-head comparative trials among GLP1RAs urged the demand for an indirect comparison of these agents. Therefore, this study was conducted to indirectly compare the CV safety and mortality effects among different GLP1RAs in patients with T2DM using network meta-analysis (NMA).
Methods
Medline was searched to identify GLP1RA CVOTs to date. The outcomes of interest were CV death, myocardial infarction (IM), stroke, and death from any cause. An NMA with binomial likelihood logit link model was used for the binary outcomes. We conducted both stock-still effects and random effects models for each upshot, and selected the all-time model based on the deviance information and the average posterior residual deviance. This NMA was reported in accord with the preferred reporting items for systematic reviews and meta-analyses (PRISMA-NMA).
Results
A total of vii GLP1RA CVOTs were included having 56,004 patients. The NMA results showed that oral semaglutide was statistically amend than exenatide (OR 0.47, 95% CI 0.21–0.99), dulaglutide (OR 0.46, 95% CI 0.20–0.97), albiglutide (OR 0.45, 95% CI 0.19–0.97), lixisenatide (OR 0.43, 95% CI 0.19–0.92) in reducing CV death events. No significant differences were detected between most of the treatments regarding reducing expiry from any cause, MI and stroke events. The ranking results showed that oral semaglutide had the highest probability to be ranked first (> xc%) in reducing CV expiry and death from any cause. Moreover, one time weekly semaglutide had the highest probability to exist ranked commencement in reducing MI and stroke events.
Conclusion
The GLP1RAs have shown meaning benefits in terms of CV rubber. The indirect comparison and ranking probability results have shown that i weekly semaglutide and oral semaglutide seems to exist the preferred option in patients with T2DM and established or at high risk of CVD. This result can assistance health care providers, pharmacy and therapeutics committees in hospitals, and insurance companies when deciding which GLP1RA to start or add to their formulary.
Background
Diabetes mellitus is a major health status that affects more than than 382 one thousand thousand adults worldwide, and this number is expected to achieve 592 million past 2035 [1]. Patients with diabetes are at a higher hazard of developing cardiovascular (CV) complications, which tin can be fatal. Cardioprotective effects of some antidiabetic drugs, along with the improvement in the provided intendance, has led to the reduction in the charge per unit of CV events over the years. However, the rate of the CV events remains significantly higher in diabetic patients than in the general population [2].
Glucagon like peptide-one receptor agonist (GLP1RA) based therapies have been in utilize since 2005 and GLP1RAs have been extensively studied for the treatment of type 2 diabetes mellitus (T2DM). Near of the GLP1RAs, including lixisenatide (short-acting) and dulaglutide (long-acting), are administered every bit a subcutaneous injection either once daily or weekly [3]. Recently, the first oral conception of a GLP1RA, daily oral semaglutide, was approved by the US food and drug administration (FDA) [three]. GLP1RAs are classified based on their structure equally either exendin-4 based (e.g. lixisenatide and exenatide) or homo GLP-1 analogue (due east.g. liraglutide, dulaglutide, semaglutide and albiglutide) [three, iv]. GLP1RAs act past increasing insulin secretion, decreasing glucagon secretion, and improving satiety. They have also been shown to have efficacy in reducing body weight and take potential antiatherothrombotic effects [3, 5].
In 2008, the US FDA mandated that all novel antidiabetic drugs must be evaluated by manufacturers to assess their CV safety profile [6]. Since then, several GLP1RA cardiovascular effect trials (CVOTs) take been conducted. To engagement, there are a total of seven GLP1RA CVOTs published [vii,8,ix,ten,11,12,13], and one trial is ongoing [14]. The trials have shown that GLP1RAs have varying degrees of CV rubber and recent meta-analyses have shown that this class has a promising CV safety profile [15,16,17,18,19]. Yet, it is still not clear which GLP1RA is preferred for CV safety. Furthermore, there is a lack of whatever head-to-head comparative trials among GLP1RAs. Therefore, this written report was conducted to indirectly compare the CV safety and bloodshed effects among dissimilar GLP1RAs in patients with T2DM using network meta-analysis (NMA).
Methods
Literature search, pick criteria and risk of bias
PubMed was searched from inception to November 2019 with the aim to identify GLP1RA CVOTs. Published randomized double blind placebo-controlled studies that have addressed the CV safety of GLP1RA as their principal outcomes were targeted. The outcomes of interest were major adverse cardiovascular events (MACE; divers as the composite endpoint of CV mortality, nonfatal myocardial infarction (MI) and nonfatal stroke termed three-bespeak MACE). In addition to MACE, decease from any cause, stroke, nonfatal stroke, MI, nonfatal MI and hospitalizations for eye failure (HF) outcomes were included. The search terms used were "glucagon like peptide-1," "GLP-1 receptor agonist," "liraglutide," "semaglutide," "albiglutide," "exenatide," "stroke," "nonfatal stroke," "myocardial infarction," "nonfatal myocardial infarction," "heart failure," "CV decease," and "CV mortality." Results were further limited to randomized controlled trials and English language. Published data were extracted from the published studies by ii authors (OMA and OAA) independently, and reviewed past a third i (OSA). The risk of bias was assessed using the Cochrane risk of bias cess tool [20].
Data synthesis and analysis
A Bayesian NMA, using binomial likelihood with logit link model and non-informative prior distribution, was used for the analysis of dichotomous outcomes. Fixed and random effects models were conducted for each effect, and then the most suitable model based on the deviance information and the average posterior residual deviance was selected. We ran inconsistency models to appraise the consistency using the residuals in inconsistency plots. Analysis was conducted using WinBUGS i.4.iii (MRC Biostatistics Unit; Cambridge, Great britain) through NetMetaXL 1.6.ane (Canadian Agency for Drugs and Technologies in Wellness; Ottawa, Canada) [21]. Nosotros evaluated the possible covariates that might not exist similar between the trials using mate-regression analyses which were performed with GeMTC GUI package [22]. The NMA was reported according to the argument of preferred reporting items for systematic reviews and meta-analyses involving network meta-assay (PRISMA-NMA) in Additional file 1: Table S1 [23].
Results
Search results and study characteristics
A full of 79 studies were identified in the systematic search, and afterwards title and abstruse screening a total of seven GLP1RA CVOTs were included, the flowchart of included and excluded studies was illustrated in Additional file two: Figure S1. The included seven studies were as follow, ELIXA (lixisenatide), LEADER (liraglutide), SUSTAIN-6 (semaglutide), EXSCEL (exenatide), Harmony outcomes (albiglutide), REWIND (dulaglutide) and PIONEER-6 (oral semaglutide) [7,8,9,x,11,12,13]. All the studies accept compared the CV safety of one of the GLP1RAs to placebo, both as added on therapy to the standard of intendance, as shown in Tabular array i. The PIONEER-vi trial was the simply trial that included an oral conception that was administered once daily (semaglutide), whereas all the remaining trials included injectable formulations. In ELIXA and LEADER trials, the drugs were administered once daily (i.eastward. lixisenatide and liraglutide) and in the residuum, once weekly (i.e. semaglutide, albiglutide, exenatide and dulaglutide).
The studies included a total of 56,004 patients with T2DM who had either established CVD or run a risk factors for CVD. The sample size in the trials ranged from 3183 patients in the PIONEER-half-dozen trial to 14,752 patients in the EXSCEL trial. The mean historic period of participants ranged from 59.9 to 66.2 years, with the REWIND trial including older patients. The median follow-upwardly ranged from one.3 years (PIONEER-vi) to five.iv years (REWIND). The ELIXA trial included but patients with contempo acute coronary syndrome. Whereas the remaining trials included varying percentages of patients with established CVD, for example 31% of patients in the REWIND trial had established CVD. The use of sodium glucose cotransporter-2 inhibitors (SGLT2is) varied among the trials; the Harmony outcomes and PIONEER-6 trials had the highest apply. Lastly, the use of statins ranged from 66 to 92% of patients in the included trials (Table 1). The network plot is presented in Boosted file three: Figure S2, the adventure of bias assessment showed that all the included trials had a low risk of bias equally presented in Additional file 4: Table S2, and at that place were no signs of inconsistency between the trials in the inconsistency plots for fixed models as demonstrated in Boosted file 5: Effigy S3.
MACE
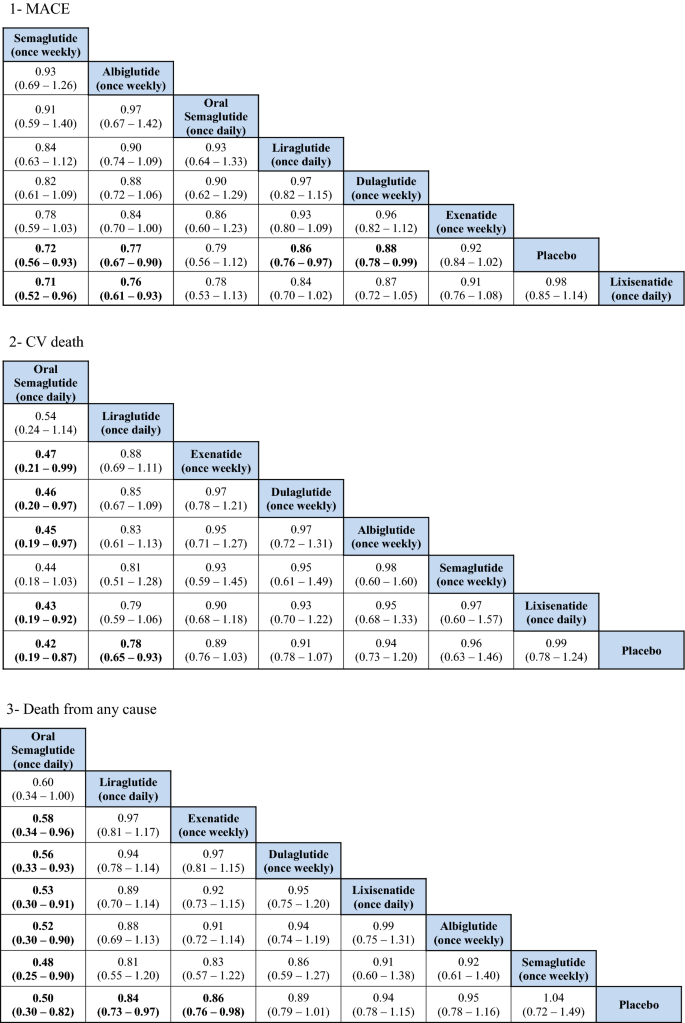
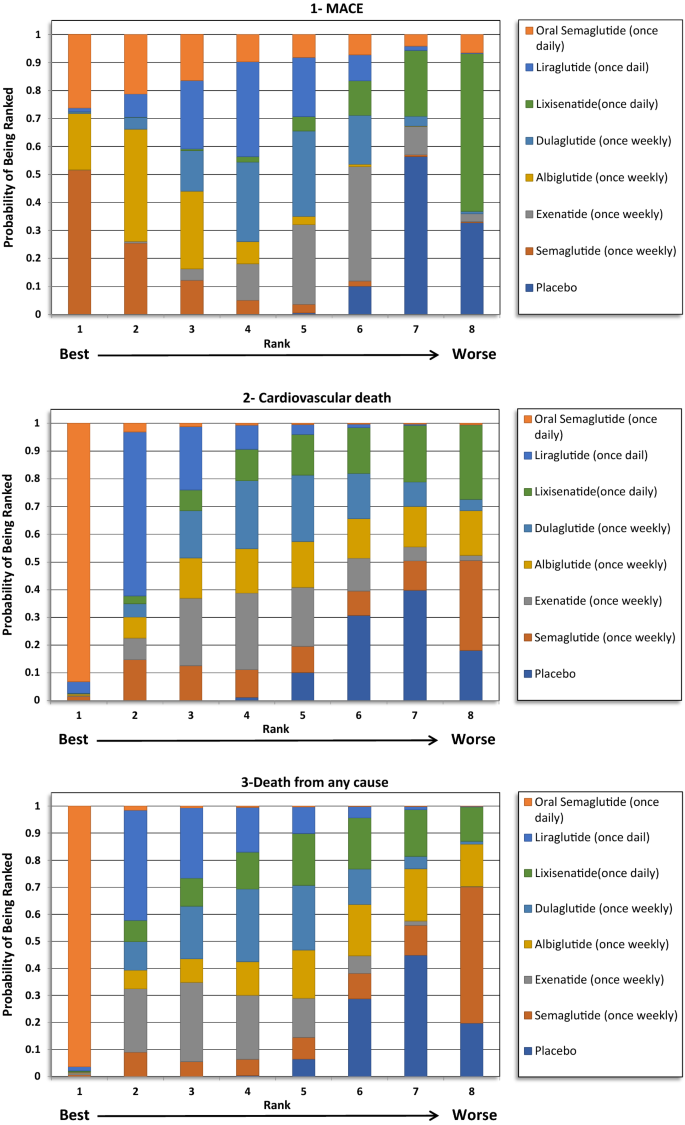
The indirect comparison showed a statistically pregnant reduction in MACE following the employ of weekly administered semaglutide compared to lixisenatide [odds ratio (OR) 0.71, 95% confidence intervals (CI) 0.52–0.96], and albiglutide compared to lixisenatide (OR 0.76, 95% CI 0.61–0.93). Moreover, the weekly injections of semaglutide, albiglutide, liraglutide (daily) and dulaglutide showed a statistically greater reduction in MACE when compared to placebo. The ranking results showed that weekly administration of semaglutide had the highest probability of beingness ranked first at 52% followed by oral semaglutide at 26% and albiglutide was ranked 3rd at 20%. The indirect comparing of GLP1RAs is presented in Fig. 1, the ranking probability is presented in Fig. 2.

Network meta-analysis results of GLP1RAs and placebo using fixed effect model
Ranking probability of GLP1RAs (Fixed Effects Rankogram)
CV death
The indirect comparing showed that oral semaglutide significantly reduced CV mortality compared with exenatide (OR 0.47, 95% CI 0.21–0.99), dulaglutide (OR 0.46, 95% CI 0.20–0.97), albiglutide (OR 0.45, 95% CI 0.19–0.97), and lixisenatide (OR 0.43, 95% CI 0.19–0.92). Moreover, oral semaglutide had the highest probability of being ranked first (> 90%) in reducing CV bloodshed in the rankogram comparing.
Death from any cause
The indirect comparison showed that oral semaglutide significantly reduced death from any cause than all the GLP1RAs except liraglutide. Therefore, oral semaglutide had the highest probability of being ranked first at 96% in the rankogram comparison.
Total MI (fatal and nonfatal)
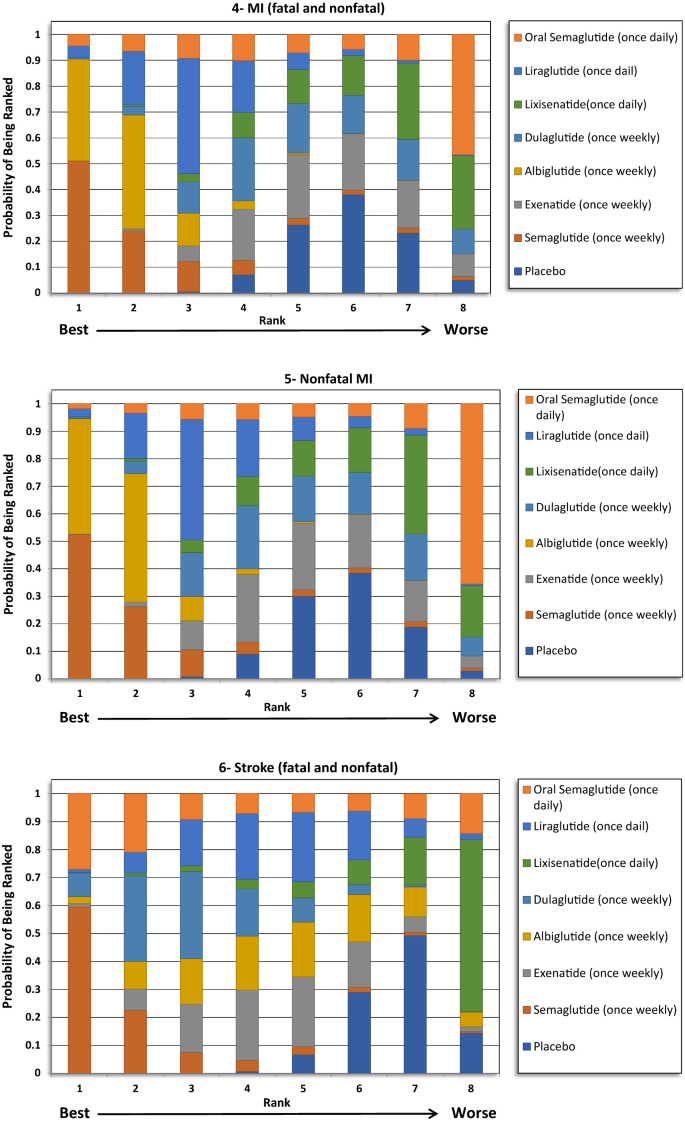
No significant differences were detected regarding total MI between the treatments except when albiglutide was compared with exenatide and lixisenatide. Albiglutide was associated with a meaning reduction in MI events compared to exenatide (OR 0.76, 95% CI 0.60–0.96) and lixisenatide (OR 0.72, 95% CI 0.55–0.93). The ranking results showed that weekly assistants of semaglutide was ranked first at 51%, followed past albiglutide at approximately 40% in the rankogram comparison.
Nonfatal MI
Similar to the results in total MI issue, albiglutide was associated with a meaning reduction in nonfatal MI compared to exenatide (OR 0.76, 95% CI 0.6–0.97) and lixisenatide (OR 0.72, 95% CI 0.55–0.93). Even so, weekly administration of semaglutide had the highest probability of being ranked commencement at approximately 52%, followed by albiglutide at 42%.
Total stroke (fatal and nonfatal)
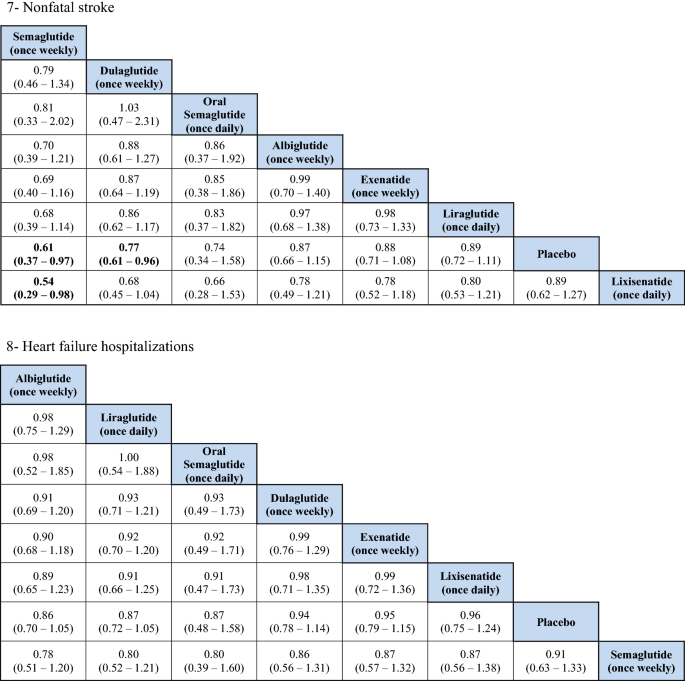
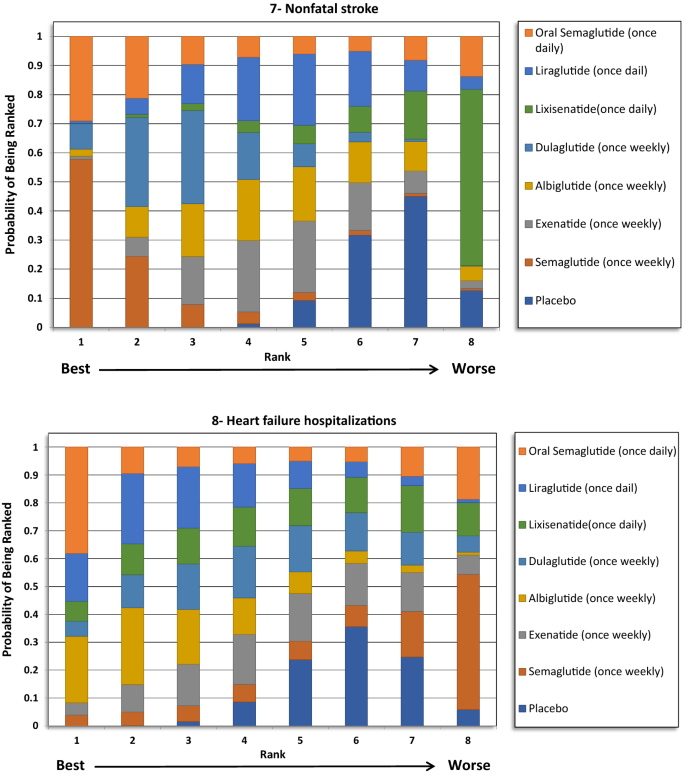
For total stroke events (fatal and nonfatal), no significant differences were identified between the treatments, except when the weekly administered semaglutide was compared to lixisenatide (OR 0.54, 95% CI 0.37–0.97). Moreover, ranking probability showed that weekly administered semaglutide was ranked beginning at 59%, followed by oral semaglutide at 27%.
Nonfatal stroke
Similar to the results in total stroke outcome, no significant difference was identified betwixt the GLP1RAs, except when the weekly semaglutide was compared to lixisenatide (OR 0.54, 95% CI 0.29–0.98). Furthermore, weekly semaglutide had the highest probability of being ranked first in reducing nonfatal stroke events at 58%, followed past oral semaglutide at 29%.
Hospitalizations for HF
The indirect comparing did not show whatever significant deviation among the GLP1RAs and placebo in reducing HF related hospitalizations. The ranking results showed that oral semaglutide had the highest probability of being ranked kickoff, followed by albiglutide.
Meta-regression analyses
The results from meta-regression analyses of the result of potential covariates did not suggest any association between the covariates and the relative outcome of GLP1RAs. The 95% CrI of the interaction coefficients for the included covariates crossed the nil value (0). These findings were presented in Boosted file 6: Table S3.
Discussion
The study was conducted to indirectly compare the CV safety and mortality furnishings among different GLP1RAs in patients with T2DM using network meta-analysis (NMA), to identify the likely preferred agent with respect to CV safety. The report did not find whatever significant deviation between GLP1RAs in reducing expiry from any cause, MI and stroke events. However, the ranking results showed that oral semaglutide had the highest probability to be ranked first (> 90%) in reducing CV death and death from whatever cause, while once weekly semaglutide had the highest probability to be ranked get-go in reducing MI and stroke events.
Several meta-analyses were performed to compare the CV safety of GLP1RAs. Furthermore, NMA was besides performed to explore the CV safety of GLP1RAs in comparing with other hypoglycemic drugs such as SGLT2is [24,25,26]. Five meta-analyses were conducted including information from all seven GLP1RAs CVOTs published to date, Kristensen et al. [xv], Giugliano et al. [xvi], Mannucci et al. [17], Marsico et al. [18], and Zhu et al. [19]. The results from these 5 meta-analyses were summarized in Additional file 7: Table S4. In the Kristensen et al. meta-analysis, GLP1RAs was associated with a 12% reduction in the iii-point MACE (hazard ratio (HR) 0.88, 95% CI 0.82–0.94) with moderate heterogeneity reported [15]; the results from the other four meta-analyses were very similar to that of Kristensen et al. [16,17,18,19]. Prior to the improver of REWIND and PIONEER-vi trials data, the reduction ranged between 10% (Hour 0.ninety, 95% CI 0.82–0.99) and 13% (OR 0.87, 95% CI 0.82–0.93) as reported past Bethel et al. and Fei et al. meta-analyses [27, 28]. The subgroup assay in the meta-assay past Kristensen et al. showed that the reduction in MACE associated with GLP1RAs was regardless of the median follow-upwards of the studies, body mass index, and HbA1c at baseline. However, no pregnant reduction in MACE was seen with daily medications, patients with no established CVD (primary prevention), or with exendin-4 based GLP1RAs. When considering the individual CVOTs, MACE was significantly reduced in the LEADER (liraglutide) [eight], SUSTAIN-6 (semaglutide once weekly) [9], Harmony outcomes (albiglutide) [xi], and REWIND (dulaglutide) trials [12]. In the current NMA, the indirect comparisons did non bear witness any meaning differences between these drugs when indirectly compared to each other with respect to the MACE outcome. Nevertheless, weekly assistants of semaglutide was associated with a 29% reduction in MACE events compared to lixisenatide (OR 0.71, 95% CI 0.52–0.96), and albiglutide was associated with a 24% reduction in MACE events compared to lixisenatide (OR 0.76, 95% CI 0.61–0.93). These results have the potential to support the case that human based GLP1RAs are more probable to exist associated with a significant reduction in MACE compared to exendin-4 based GLP1RAs. Notwithstanding, it is important to note that, in the subgroup assay previously reported by Kristensen et al. the exendin-iv based drugs showed significant heterogeneity, which might advise the beingness of differences in the trial'south populations and design, more than than the chemical construction. Nevertheless, in our analysis, the weekly semaglutide was the preferred agent among all of the GLP1RAs based on ranking probability results.
When examining the private components of MACE, a significant reduction in CV mortality (12–xiii%) and stroke (16–17%) was consistently reported for GLP1RAs, with no heterogeneity, in the five aforementioned meta-analyses. However, the reduction in the MI events (8–9%) was not as robust as the results for the CV mortality or the stroke outcomes, as this was only significant in three of the v meta-analyses [15,16,17,18,19]. Moreover, the reduction in MI events was meaning in two CVOTs, namely the LEADER (liraglutide) and Harmony outcomes (albiglutide) trials. All the same, the remaining trials did non testify a pregnant reduction. The CV bloodshed consequence was largely driven past the pregnant reduction that was seen in the LEADER (liraglutide) and PIONEER-6 (oral semaglutide) trials. Avgerinos et al. found oral semaglutide to be superior to placebo in term of CV mortality (OR 0.55, 95% CI 0.31–0.98), just that was non significant when oral semaglutide was compared to other antidiabetic agents, including liraglutide [29]. In our analysis, the indirect comparison showed that oral semaglutide was associated with a greater reduction in CV mortality events compared with exenatide, lixisenatide, albiglutide and dulaglutide, but not when compared with liraglutide or once weekly semaglutide. Nevertheless, the ranking results showed that oral semaglutide was the preferred GLP1RA by more than 90% probability. Furthermore, the indirect comparison did not provide pregnant differences in relation to MI and stroke reduction between the GLP1RAs. Although, weekly semaglutide had the highest probability of being ranked first for both outcomes.
Death from whatever crusade was also reported to be significantly reduced by GLP1RAs. The reduction ranged between ten and 12% in four of the five meta-analyses, with no significant heterogeneity reported [fifteen,xvi,17,xviii], and the last meta-analysis did not evaluate this outcome [19]. Such reduction was largely driven by the PIONEER-6 (oral semaglutide) trial, and that was previously reflected in the Avgerinos et al. meta-analysis when they compared oral semaglutide to placebo (OR 0.58, 95% CI 0.37–0.92) [29]. In our analysis, oral semaglutide significantly reduced the deaths from any cause when indirectly compared with all other GLP1RAs, except liraglutide which was besides reported past the Avgerinos et al. meta-analysis [29]. Moreover, oral semaglutide was ranked commencement in comparison with other GLP1RAs with a probability of more than than ninety%. This event is very promising for the get-go oral GLP1RAs. Although, information technology cannot be entirely explained given the lack of heterogeneity. PIONEER-6 was the shortest trial in terms of duration and included patients with a longer duration of T2DM at baseline (fourteen.nine years) and had the highest utilise of SGLT2is at 9%. Although CV mortality and all-cause mortality events were significantly reduced in the PIONEER-half dozen trial, the reduction in MACE did non attain statistical significance [thirteen].
For the HF related hospitalization, the use of SGLT2is was previously plant to be associated with the near cardioprotective effect in patients with HF, or even at risk of having HF, compared to all other antidiabetic classes, including GLP1RA [28, xxx]. Nonetheless, different previously reported meta-analyses, Kristensen et al., Giugliano et al., and Fei et al. were the get-go to report a significant reduction in the rate of HF related hospitalizations, and afterwards on Marsico et al. and Zhu et al. reported the same finding in their near recent meta-analyses. GLP1RAs were associated with eight–xiii% reduction in the rate of HF related hospitalizations, with no heterogeneity [15, 16, 18, xix, 28]. The Harmony outcomes (albiglutide) trial was the but trial to testify statistical significance regarding reduction in HF related hospitalizations. In our analysis, no meaning difference was found amidst the GLP1RAs, including albiglutide. However, it is important to note that albiglutide is no longer available in the market, as per the decision made past GlaxoSmithKline in 2017 [31]. Regardless of the pocket-size clinical significance of this effect to patients with established or at take a chance of HF, knowing that GLP1RAs may take a modest positive outcome is very assuring for the utilise of GLP1RAs for combined drug therapy with SGLT2is or as an culling to SGLT2is in patients with T2DM and established or at run a risk of HF. The Fei et al. meta-assay [28] indicated the superiority of GLP1RAs over dipeptidyl peptidase-4 inhibitors (DPP-4i) in term of HF related hospitalization, this finding negated information from an observational cohort study that was previously conducted by Dawwas et al. [32] using existent world information. Therefore, more research is needed to assess the superiority of GLP1RAs and SGLT2is over other classes of antidiabetic agents in real-world practice.
The run a risk of CVD, CV bloodshed, and expiry from whatsoever cause varies amid patients with T2DM based on age, HbA1c level, history of CVD, and the duration of diabetes [33,34,35]. The utilise of GLP1RA, such equally once weekly semaglutide in the SUSTAIN 6 trial, was found to be an contained predictor for the reduction in the rate of MACE, CV mortality, nonfatal MI, and nonfatal stroke [36]. This was supported by the findings from the meta-regression in the electric current written report, equally all the CV benefits were contained of age, elapsing of diabetes, mean HbA1c level, and the beingness of CVD at baseline. This indicates that the CV protective effect for GLP1RA can be seen in most patients with T2DM; therefore, all patients that have established or at high take a chance of CVD would do good from being initiated on GLP1RA regardless of their age, duration of diabetes, and HbA1c level. Moreover, when using i of the GLP1RAs, the addition of some other agent with CV protective event, such equally one of the SGLT2i, tin contribute to even better CV outcomes in these patients [37]. Therefore, in patients with established or at loftier risk of CVD when boosted therapy is needed for amend control of diabetes, agents with CV protective issue should be considered.
In light of the recent results of CVOTs, the diabetes guidelines have changed their recommendations regarding T2DM handling. This change was heavily influenced past the CV consequence of drugs regardless of the glycemic effect. The nearly contempo American Diabetes Association (ADA) guidelines of 2020 recommend the use of GLP1RAs as an add-on to metformin or equally a first line therapy for selected patients who cannot tolerate metformin [iii]. Moreover, the use of the long-acting GLP1RAs is recommended prior to the addition of basal insulin. For patients with established or at loftier take chances of CVD, administration of GLP1RAs and SGLT2is—that have demonstrated CVD benefit—should be undertaken regardless of glycemic command. For those with HF (especially with reduced ejection fraction), the apply of SGLT2is is preferred over other drug classes since canagliflozin, dapagliflozin, and empagliflozin demonstrated HF benefit. The ADA guidelines recommend the use of GLP1RAs as an alternative to those who cannot tolerate SGLT2is [3]. This recommendation is consequent with the recent meta-analyses that has shown a statistically significant reduction in HF hospitalizations with GLP1RAs [fifteen, 16, 18, 19, 28].
Limitations
In this paper, other safe issues were not addressed, such as retinopathy. However, recent meta-analysis did not testify meaning increment in retinopathy events with the use of GLP1RAs [fifteen]. At that place are differences in terms of populations amidst the trials that cannot exist controlled by the NMA blueprint. However, we explored key variables in the baseline characteristics of patients included in the trials using the meta-regression methods that did not suggest major variations between these populations. Moreover, the included trials used a composite chief endpoint (MACE) to achieve sufficient ability but some components of MACE that drive the outcome number may differ from study to another. Therefore, the results should exist interpreted charily. For stroke events, some trials reported either nonfatal events simply or total stroke events only. Although albiglutide is no longer bachelor in the market, given that albiglutide was included in previously reported meta-analyses, albiglutide data was included in this NMA. Given that the researchers are very familiar with the most contempo piece of work that were done and published in this field, an all-encompassing literature search on multiple databases was not deemed necessary, and therefore was not conducted. Lastly, when relying on the findings from this enquiry the reader should acknowledge that this report did non focus on another important decision-making parameters, such equally toll of medications, weight reduction, glycemic command and other condom issues. Therefore, these aspects would need to be reviewed from other research, and if this information are not bachelor then information technology would exist an area for time to come research.
Conclusion
The GLP1RAs have shown meaning benefits in terms of CV safety. While utilizing information from the CVOTs to date, the indirect comparison and ranking probability results have shown that i weekly semaglutide and oral semaglutide seems to be the preferred option in patients with T2DM and established or at high gamble of CVD. This result can aid wellness care providers, pharmacies and therapeutics committees in hospitals, and insurance companies when deciding which GLP1RA to kickoff or add to their formulary.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its boosted information files].
Abbreviations
- CV:
-
Cardiovascular
- CVD:
-
Cardiovascular disease
- GLP1RA:
-
Glucagon similar peptide-1 receptor agonist
- T2DM:
-
Type 2 diabetes mellitus
- CVOT:
-
Cardiovascular outcome trial
- NMA:
-
Network meta-analysis
- MI:
-
Myocardial infarction
- MACE:
-
Major adverse cardiovascular events
- HF:
-
Heart failure
- SGLT2i:
-
Sodium glucose transporter-two inhibitor
References
-
Guariguata Fifty, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. https://doi.org/10.1016/j.diabres.2013.11.002.
-
Rawshani A, Rawshani A, Franzén S, Eliasson B, Svensson A-M, Miftaraj M, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. Due north Engl J Med. 2017;376:1407–18. https://doi.org/10.1056/NEJMoa1608664.
-
American Diabetes Association. Standards of medical care in diabetes—2020: 9. Pharmacologic approaches to glycemic treatment. Diabetes Intendance. 2020;43(Suppl 1):S98–110. https://doi.org/x.2337/dc19-s009.
-
Miñambres I, Pérez A. Is in that location a justification for classifying GLP-ane receptor agonists as basal and prandial? Diabetol Metab Syndr. 2017;9:6. https://doi.org/10.1186/s13098-017-0204-half-dozen.
-
Sposito AC, Berwanger O, de Carvalho LSF, Saraiva JFK. GLP-1RAs in type ii diabetes: mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc Diabetol. 2018;17:157. https://doi.org/10.1186/s12933-018-0800-2.
-
Food and Drug Assistants (FDA). Guidance for industry: Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to care for blazon two diabetes. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diabetes-mellitus-evaluating-cardiovascular-risk-new-antidiabetic-therapies-treat-type-two-diabetes. Accessed on thirty January 2020.
-
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type ii diabetes and astute coronary syndrome. N Engl J Med. 2015;373:2247–57. https://doi.org/ten.1056/NEJMoa1509225.
-
Marso SP, Daniels GH, Dark-brown-Frandsen One thousand, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. https://doi.org/10.1056/NEJMoa1603827.
-
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar Eastward, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type two diabetes. N Engl J Med. 2016;375:1834–44. https://doi.org/x.1056/NEJMoa1607141.
-
Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of one time-weekly exenatide on cardiovascular outcomes in type two diabetes. Northward Engl J Med. 2017;377:1228–39. https://doi.org/10.1056/NEJMoa1612917.
-
Hernandez AF, Light-green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–29. https://doi.org/10.1016/S0140-6736(18)32261-X.
-
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–30. https://doi.org/10.1016/s0140-6736(xix)31149-3.
-
Husain M, Birkenfeld AL, Donsmark Chiliad, Dungan Grand, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type ii diabetes. North Engl J Med. 2019;381:841–51. https://doi.org/10.1056/NEJMoa1901118.
-
A written report to evaluate cardiovascular outcomes in patients with type two diabetes treated with ITCA 650. CinicalTrials.gov website. https://clinicaltrials.gov/ct2/show/NCT01455896. Accessed 5 February 2020.
-
Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, bloodshed, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–85. https://doi.org/10.1016/s2213-8587(19)30249-ix.
-
Giugliano D, Maiorino MI, Bellastella M, Longo M, Chiodini P, Esposito G. GLP-one receptor agonists for prevention of cardiorenal outcomes in type ii diabetes: an updated meta-analysis including the REWIND and PIONEER 6 trials. Diabetes Obes Metab. 2019;21:2576–lxxx. https://doi.org/10.1111/dom.13847.
-
Mannucci Due east, Dicembrini I, Nreu B, Monami M. Glucagon-similar peptide-1 receptor agonists and cardiovascular outcomes in patients with and without prior cardiovascular events: an updated meta-analysis and subgroup analysis of randomized controlled trials. Diabetes Obes Metab. 2019;22:203–xi. https://doi.org/10.1111/dom.13888.
-
Marsico F, Paolillo S, Gargiulo P, Bruzzese D, Dell'Aversana South, Esposito I, et al. Effects of glucagon-like peptide-ane receptor agonists on major cardiovascular events in patients with Type ii diabetes mellitus with or without established cardiovascular disease: a meta-analysis of randomized controlled trials. Eur Middle J. 2020. https://doi.org/ten.1093/eurheartj/ehaa082.
-
Zhu J, Yu X, Zheng Y, Li J, Wang Y, Lin Y, et al. Association of glucose-lowering medications with cardiovascular outcomes: an umbrella review and bear witness map. Lancet Diabetes Endocrinol. 2020;viii:192–205. https://doi.org/10.1016/S2213-8587(19)30422-X.
-
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing take a chance of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928.
-
Brown S, Hutton B, Clifford T, Coyle D, Grima D, Wells 1000, et al. A Microsoft-Excel-based tool for running and critically appraising network meta-analyses–an overview and application of NetMetaXL. Syst Rev. 2014;3:110. https://doi.org/ten.1186/2046-4053-3-110.
-
van Valkenhoef 1000, Lu Yard, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-assay. Res Synth. Methods. 2012;3:285–99. https://doi.org/x.1002/jrsm.1054.
-
Hutton B, Catalá-López F, Moher D. The PRISMA argument extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med Clin (Engl Ed). 2016;147:262–6. https://doi.org/10.1016/j.medcle.2016.ten.003.
-
Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, et al. Association between apply of sodium-glucose cotransporter 2 inhibitors, glucagon-similar peptide ane agonists, and dipeptidyl peptidase 4 inhibitors with all-cause bloodshed in patients with blazon 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580–91. https://doi.org/10.1001/jama.2018.3024.
-
Hussein H, Zaccardi F, Khunti G, Seidu Southward, Davies MJ, Gray LJ. Cardiovascular efficacy and safety of sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists: a systematic review and network meta-analysis. Diabetic Med. 2019;36:444–52. https://doi.org/x.1111/dme.13898.
-
Wu South, Cipriani A, Yang Z, Yang J, Cai T, Xu Y, et al. The cardiovascular event of incretin-based therapies among type 2 diabetes: a systematic review and network meta-analysis. Expert Opin Drug Saf. 2018;17:243–9. https://doi.org/10.1080/14740338.2018.1424826.
-
Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, et al. Cardiovascular outcomes with glucagon-like peptide-ane receptor agonists in patients with type 2 diabetes: a meta-assay. Lancet Diabetes Endocrinol. 2018;6:105–13. https://doi.org/ten.1016/s2213-8587(17)30412-6.
-
Fei Y, Tsoi MF, Cheung BMY. Cardiovascular outcomes in trials of new antidiabetic drug classes: a network meta-analysis. Cardiovasc Diabetol. 2019;18:112. https://doi.org/10.1186/s12933-019-0916-z.
-
Avgerinos I, Michailidis T, Liakos A, Karagiannis T, Matthews DR, Tsapas A, et al. Oral semaglutide for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22:335–45. https://doi.org/ten.1111/dom.13899.
-
Yang D-Y, He X, Liang H-W, Zhang S-Z, Zhong X-B, Luo C-F, et al. Comparative outcomes of heart failure among existent classes of anti-diabetic agents: a network meta-analysis of 171,253 participants from 91 randomized controlled trials. Cardiovasc Diabetol. 2019;18:47. https://doi.org/ten.1186/s12933-019-0853-ten.
-
Yu M, Benjamin MM, Srinivasan S, Morin EE, Shishatskaya EI, Schwendeman SP, et al. Battle of GLP-1 commitment technologies. Adv Drug Deliv Rev. 2018;130:113–30. https://doi.org/10.1016/j.addr.2018.07.009.
-
Dawwas GK, Smith SM, Park H. Risk of centre failure hospitalization among users of dipeptidyl peptidase-4 inhibitors compared to glucagon-like peptide-one receptor agonists. Cardiovasc Diabetol. 2018;17:102. https://doi.org/10.1186/s12933-018-0746-4.
-
Raghavan S, Vassy JL, Ho YL, Song RJ, Gagnon DR, Cho Thousand, et al. Diabetes mellitus–related all-crusade and cardiovascular mortality in a national cohort of adults. Am Middle J. 2019;8:e011295. https://doi.org/10.1161/JAHA.118.011295.
-
Zhang H, Qin L, Sheng C-S, Niu Y, Gu H, Lu S, et al. ASCVD risk stratification modifies the outcome of HbA1c on cardiovascular events among patients with type two diabetes mellitus with bones to moderate take a chance. BMJ Open Diabetes Res Care. 2020;8:e000810. https://doi.org/x.1136/bmjdrc-2019-000810.
-
Play a joke on CS, Sullivan Fifty, D'Agostino RB, Wilson PWF. The pregnant effect of diabetes duration on coronary center disease mortality. Diabetes Care. 2004;27:704–8. https://doi.org/10.2337/diacare.27.iii.704.
-
Leiter LA, Bain SC, Hramiak I, Jódar E, Madsbad S, Gondolf T, et al. Cardiovascular take chances reduction with once-weekly semaglutide in subjects with type 2 diabetes: a post hoc analysis of gender, historic period, and baseline CV run a risk contour in the SUSTAIN half-dozen trial. Cardiovasc Diabetol. 2019;18:73. https://doi.org/10.1186/s12933-019-0871-eight.
-
Clegg LE, Penland RC, Bachina Due south, Boulton DW, Thuresson M, Heerspink HJL, et al. Effects of exenatide and open up-label SGLT2 inhibitor handling, given in parallel or sequentially, on bloodshed and cardiovascular and renal outcomes in type 2 diabetes: insights from the EXSCEL trial. Cardiovasc Diabetol. 2019;18:138. https://doi.org/10.1186/s12933-019-0942-10.
Acknowledgements
The authors would similar to extend their appreciation to Rex Saud University for funding this piece of work through the Researcher Supporting Project (RSP-2019/77), King Saud University, Riyadh, Saudi Arabia. They would as well like to thank Editage® for English language linguistic communication editing of this manuscript.
Funding
The author (OAA) received a fund from the Researcher Supporting Project (RSP-2019/77), Male monarch Saud University, Riyadh, Saudi Arabia, to back up the publication of this article. The funding bureau had no role in designing the written report, conducting the analysis, interpreting the data or writing the manuscript.
Author data
Affiliations
Contributions
OMA designed the study, extracted the data, and was a major contributor in writing the manuscript. OAA extracted the information and contributed in writing the manuscript. OSA reviewed the extracted data for the analysis and the tables for the results, and contributed in writing the manuscript. ARA participated in designing the study, analyzed the data, and produced the tables and figures. MSA reviewed the analysis and contributed in writing the manuscript. All authors read and approved the concluding manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicative.
Consent for publication
Not applicative.
Competing interests
The authors declare that they have no competing interests.
Additional data
Publisher'southward Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open up Access This commodity is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, accommodation, distribution and reproduction in any medium or format, equally long as you lot give appropriate credit to the original author(s) and the source, provide a link to the Artistic Commons licence, and indicate if changes were made. The images or other 3rd party textile in this article are included in the commodity'south Creative Eatables licence, unless indicated otherwise in a credit line to the material. If cloth is not included in the article'southward Creative Eatables licence and your intended apply is not permitted past statutory regulation or exceeds the permitted utilize, y'all will demand to obtain permission directly from the copyright holder. To view a re-create of this licence, visit http://creativecommons.org/licenses/by/iv.0/. The Creative Eatables Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made bachelor in this commodity, unless otherwise stated in a credit line to the data.
Reprints and Permissions
Almost this article
Cite this article
Alfayez, O.Yard., Almohammed, O.A., Alkhezi, O.S. et al. Indirect comparison of glucagon like peptide-one receptor agonists regarding cardiovascular rubber and mortality in patients with type 2 diabetes mellitus: network meta-analysis. Cardiovasc Diabetol 19, 96 (2020). https://doi.org/10.1186/s12933-020-01070-z
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/s12933-020-01070-z
Keywords
- Cardiovascular disease
- Diabetes mellitus
- Type 2
- Meta-analysis
- Myocardial infarction
- Stroke
- Center failure
Source: https://cardiab.biomedcentral.com/articles/10.1186/s12933-020-01070-z
0 Response to "Review of Headã¢â‚¬âtoã¢â‚¬âhead Comparisons of Glucagonã¢â‚¬âlike Peptideã¢â‚¬â1 Receptor Agonists"
Post a Comment